Future Unicorns
- Home
- Information Center
- Future Unicorns

About the Company
Background of Development
Baek Woo-yeol, CEO of PLCOskin, a plastic surgeon and professor at Severance Hospital of Yonsei University, is the developer of TissueDerm™, an artificial soft tissue that can be mass-produced without side effects. TissueDerm™ is a safe artificial implant medical device that is reasonably priced at approximately half the price of other products in the soft tissue reconstruction market but does not have side effects. Containing FDA-approved and safe polycaprolactone (PCL), TissueDerm™ has excellent biocompatibility and high tensile strength and is safe and easy to fixate on the body as it does not cause inflammation. Moreover, it is very appropriate for treating patients with soft tissue defects as it induces the generation of autologous fat.
About the Product and Technology
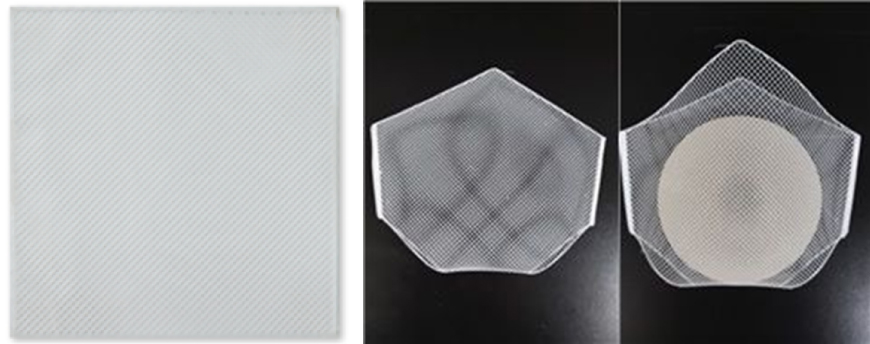
- Artificial dermis for soft tissue regeneration and silicone prosthesis cover
- Excellent bio-transplantation performance with less inflammatory response than acellular dermal matrix (ADM)
- Stable supply ensured by placing biodegradable polymers (polycaprolactone) on collagen sponge with 3D printing technology
- Biodegradable polymers remove issues related to adhesion
- Collagen used to guarantee high biocompatibility
- Unlike ADM, TissueDerm is capable of inducing the regeneration of autologous fat.
- Potentials for application in other product groups such as ligaments and abdominal walls
Competitive Edge and Business Strategy
IPR (Competitiveness)- KFDA license for medical device manufacturing -TissueDerm™
- Applied for six patents at home and abroad/Completed the registration of two patents
- Selected as a partner in a joint R&D project with Israel (KRW 2 billion)
- Selected as a EUROSTAR project of international joint R&D with Europe (KRW 2 billion)
- 2022: Selected as an innovative startup by IBK Changgong
- 2022: Selected as SBA Seoul’s promising exporter
- 2019: Selected to implement TIPS (Tech Incubator Program for startup Korea), the Ministry of SMEs and Startup’s private investment-led tech startup program
- 2019: Listed in Top 100 business in Slush-Helsinki, the world’s biggest startup event
Future Plans
In the second place, PLCOskin will explore overseas markets by utilizing its networks with US university hospitals, Japanese trade companies, and a Dutch/Israeli research company and actively participate in fairs, exhibitions, and events for finding overseas buyers.
Lastly, PLCOskin aims to improve patients' quality of life as a global biotech company in the future by increasing corporate value based on active IR and consistently attracting investments.
By Baek Woo Yeol (wbaek@plcoskin.com)
CEO, PLCOSKIN










