Current status of Industrial Clusters
- Home
- Investment Projects
- Current status of Industrial Clusters
Daegu-Gyeongbuk High-tech Medical Complex
Date
2014.09.18
Views
6432
Daegu-Gyeongbuk High-tech Medical Complex (Medivalley)
Medivalley is an advanced medical technology cluster, that is nationally designated to strategically foster a world-class pharmaceutical and medical device industries to lead the global medical market
Overview
- Location: Sinseo-dong, Dong-gu, Daegu City
- Project Period: 2009 ~ 2038 (Construction or ndustrial complex 2009~2013)
- Gross Area: 1,026,000 ㎡ (Business and R&D zones 439,000 ㎡)
- Implementer: Korea Land and Housing Corporation (LH)
- Project Cost: KRW4.6 trillion
Site Conditions
- Target Sector: Manufacturing and R&D about pharmaceuticals and advanced medical devices
- Rent: KRW 2.85 million/3.3㎡
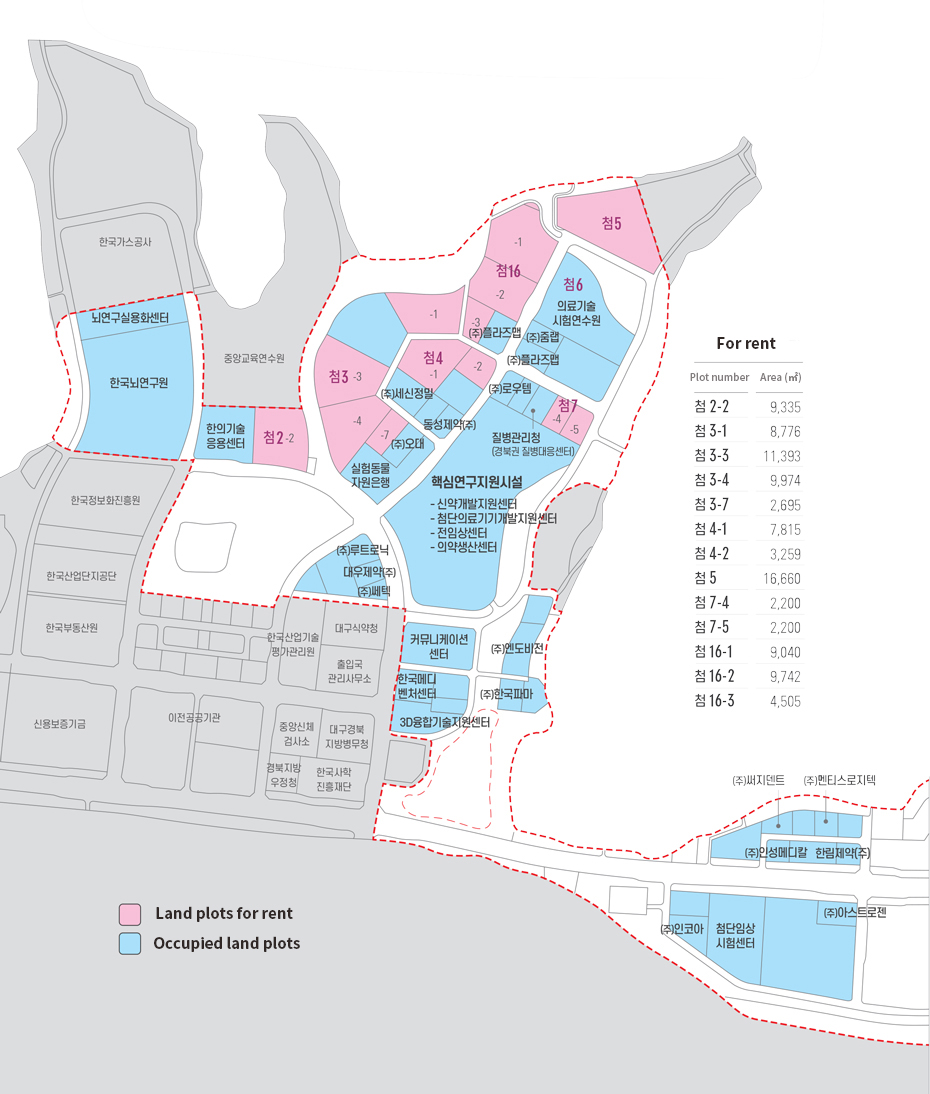
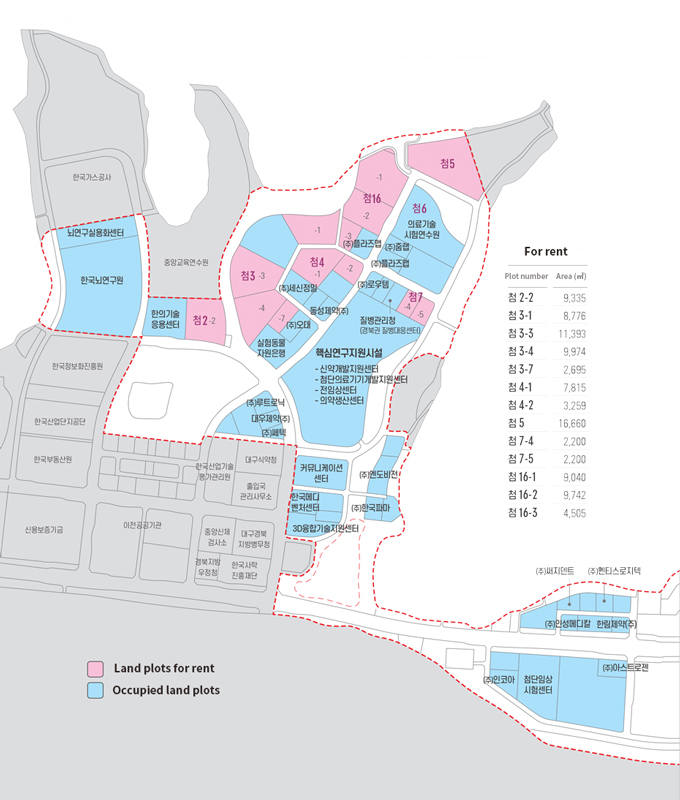
- Occupancy Rate
(As of January, 2023)
산업단지 분양실적을 나타내는 표 Land Use Planning Total Area (1,000 ㎡) Number of Tenants Occupied (1,000 ㎡) For rent (1,000 ㎡) Occupancy Rate (%) Business and R&D Zone 439 27 entities* 341 98 77.8 ※ Additional tenants: 32 entities in Korea Mediventure Center located within Daegu-Gyeongbuk High-tech Medical Complex (lot for sale), 6 entities in Communication Center (lease), 5 entities in New Drug Development Center (lease), 7 entities in 3D Convergence Technology Center (lease) - Access: Medivalley is connected with 8 express ways through Dongdaegu (East Daegu) IC, an entrance ramp to Gyeongbu Expressway
It is 5 minutes’ walk from a station of Daegu Metro line 1 - Key Feature: The best place to live and work as it is located within Daegu innovation city
The best national medical R&D cluster
(Four core infrastructures that include those for drug development and cutting-edge medical devices, Korea Brain Research Institute)
Regulatory special cases according to Special Act on the Designation and Support of High-tech Medical Complexes and Regulation-Free Special Zone on Smart Wellness
Major Tenant
- Corporation: DongSung Pharm., Lutroinc, Jamlim Pharm., Surgident, Korea Pharma
- Research Center: Daegu Gyeongbuk Medical Innovation Foundation (DGMIF), Advanced Clinical Trial Center, Korea Brain Research Institute, Laboratory Animal Resources Bank, Training Center for Medical Technology and Test, etc.
- Support Agency: Daegu Regional FDA
Target Institution
- Institutions designed for medical R&D (pharmaceuticals or medical devices)
- Institutions that create and run departments in charge of medical R&D
- Medical institutions
- R&D centers (including annex research institutes of those in charge of pharmaceuticals, medical devices, or the health and medical technology industry)